This tutorial describes superimposing structures, saving positions and sessions, and creating publication-quality images. See also: presets, tips on preparing images
The subjects are enzymes with shared structural features, as described in the papers:
T. Pons, D.G. Naumoff, C. Martinez-Fleites, and L. Hernandez, "Three Acidic Residues Are at the Active Site of a Beta-Propeller Architecture in Glycoside Hydrolase Families 32, 43, 62, and 68" Proteins 54:424 (2004).
F. Alberto, C. Bignon, G. Sulzenbacher, B. Henrissat, and M. Czjzek, "The Three-Dimensional Structure of Invertase (Beta-Fructosidase) from Thermatoga maritima Reveals a Bimodular Arrangement and an Evolutionary Relationship between Retaining and Inverting Glycosidases" J Biol Chem 279:18903 (2004).
| [images shown at 25%; click for full size] |

|

|
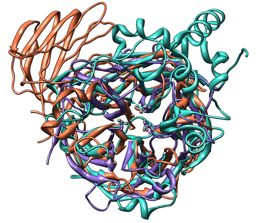
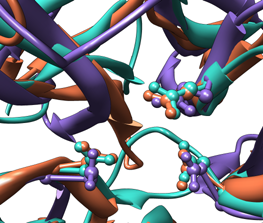
| Comparison of glycoside hydrolases from different families. Invertase (family 32) is coral, arabinanase A (family 43) is purple, and levansucrase (family 68) is turquoise. Despite differences in sequence, substrate, and catalytic mechanism, the enzymes have similar overall folds (top) and a set of highly conserved acidic residues in the active site (bottom). |
The same structures are used in the Alignments tutorial.
Glycoside hydrolases (GH) are a large, heterogeneous set of enzymes that hydrolyze bonds between sugars and other groups. Based on sequence comparisons, these enzymes have been categorized into many families; see the CAZy web site for details.
This tutorial describes the creation of images to show structural similarities among certain members of GH families 32, 43, and 68. These enzymes were grouped into different families because their relationships were not clear from sequence information alone.
The approach described on this page is only one of many possibilities. Another is to generate static renderings with shadows.
On Windows/Mac, click the chimera icon. On UNIX, start Chimera from the system prompt:
unix: chimera
A basic Chimera window should appear after a few seconds; resize it as desired. Open the Command Line (choosing Tools... General Controls... Command Line is one way).
If you have internet connectivity, structures can be obtained directly from the Protein Data Bank. Choose File... Fetch by ID from the Chimera menu. In the resulting dialog, check the PDB ID option (if it is not already checked) and the option to Keep dialog up after Fetch. Fetch the PDB structures 1uyp, 1gyd, and 1oyg, in that order, and then Close the dialog. If you do not have internet connectivity, you can download the files 1uyp.pdb, 1gyd.pdb, and 1oyg.pdb into your working directory and then open them in that order as local files (with File... Open). (Or instead of opening these structures, one could just open the Chimera session that is saved below, but that would not be very instructive as to how the session was generated.)
Move and scale the structures with the mouse in the graphics window (and optionally, the Side View) as desired throughout the tutorial.
Some salient features of the structures:
| PDB ID | enzyme | family | chains | conserved residues | ||
|---|---|---|---|---|---|---|
| 1uyp | invertase, T. maritima |
GH32 | A-F | Asp17 | Asp138 | Glu190 |
| 1gyd | arabinanase A, C. japonicus |
GH43 | B | Asp38 | Asp158 | Glu221 |
| 1oyg | levansucrase, B. subtilis |
GH68 | A | Asp86 | Asp247 | Glu342 |
First, simplify the situation by deleting unwanted (for our purposes) portions of the structures such as extra chains and solvent, and then displaying just the alpha-carbon traces.
Command: delete #0:.b-fSince a set of structurally equivalent residues is known, an efficient way to superimpose the structures is by specifying atoms to use in a least-squares fit:
Command: delete solvent
Command: chain @ca
Command: linewidth 3
Command: match #1:38.b,158.b,221.b@n@ca@c@o #0:17.a,138.a,190.a@n@ca@c@oIn fact, the chain specifications .a and .b could be omitted from these match commands because the residues would still be specified uniquely without them (other chains containing the same residue numbers in the same model have been deleted). In the general case, chain ID codes are needed to specify residues uniquely because a given residue number may occur in more than one chain.
Command: match #2:86.a,247.a,342.a@n@ca@c@o #0:17.a,138.a,190.a@n@ca@c@o
The peptide backbone atoms of the conserved residues were used to match the structures. Another possibility would have been to use all atoms of these residues, as the residue types are the same across the different structures (match requires equal numbers of atoms from the two models being matched). Model 0 (invertase) was used as the reference structure for both pairwise matches. The number of points used for fitting and the resultant RMSD values are reported in the status line (transiently) and the Reply Log.
Alternatively, matchmaker could have been used to superimpose the structures. Instead of user-specified atoms, it uses the protein sequences and secondary structure to generate alignments. Superpositions similar to those obtained above with match can be obtained with the following:
Command: matchmaker #0 #1Compared to match, matchmaker is often easier to use because structurally equivalent atoms do not need to be specified; however, it takes more time (due to the sequence alignment step) and offers less control over exactly which atoms will be used for the fit. Also, matchmaker can only be used on peptide and nucleotide chains, not other types of molecules.
Command: matchmaker #0 #2
Undisplay atoms and show the structures as ribbons:
Command: ~dispThe default ribbon style is flat. The edged and rounded styles are more three-dimensional.
Command: ribbon
Command: ribrepr roundedThe Chimera default ribbon scaling (secondary-structure-specific height and width) was used for the example images. New scalings and styles can be defined, named, and saved for later use with the Ribbon Style Editor (Tools... Depiction... Ribbon Style Editor).
The entire protein chains are shown. Although not done for the examples, ribbon can be undisplayed for parts of chains:
Command: ~ribbon #0:296-end.a
Show only the side chains of the conserved residues, in ball-and-stick form:
Command: sel #0:17.a,138.a,190.a #1:38.b,158.b,221.b #2:86.a,247.a,342.aAs explained above, the chain specifications .a and .b could be omitted from the selection command. Even though all atoms of the residues are selected, by default the backbone atoms are hidden when ribbon is shown (this can be controlled with the command ribbackbone).
Command: disp sel
Command: repr bs sel
Command: ~sel
The background can be any color, but white is often good for publication.
Command: set bg_color whiteThe background color can also be changed with the menu (Actions... Color) and in the Background preferences. Further, if system hardware permits, background transparency can be enabled in the Effects tool. Images saved with a transparent background are easier to composite with different backgrounds in image-editing applications.
Presets may also change the background color, along with several other display and rendering quality settings.
Depth cueing is shading the scene from front to back, which looks like "fog" when the depth cueing color is light. Open the Effects tool (Tools... Viewing Controls... Effects). The default depth cueing color is undefined (shown as "No"), meaning that it will automatically match the background. The amount of depth-cueing shading can be adjusted by moving the clipping planes in the Side View. Uncheck the depth cueing checkbox to remove the fog effect.
Also in Effects, turn on silhouette edges to highlight boundaries and discontinuities, then Close the tool. Edges may look jagged, but supersampling will correct these within saved images.
Color the three models three distinguishable colors.
Command: sel #0Repeat for models 1 and 2. For the example images, the colors named coral, medium purple, and turquoise were chosen for models 0, 1, and 2, respectively. Clear the selection:
Actions... Color... all colors [pick a color]
Command: ~selOpen Shininess (Tools... Viewing Controls... Shininess). Set the shininess to 128 and the brightness to 5.0, then Close the tool. Although not done for the examples, the positions, colors, and intensities of the two lighting sources in Chimera can be adjusted with the Lighting tool.
The smoothness of curved surfaces in the stick, ball-and-stick, sphere, and ribbon molecular representations can be increased by raising subdivision quality in the Effects tool (Tools... Viewing Controls... Effects). Higher values increase smoothness but may slow performance. Values of 5.0-20.0 are reasonable for saved images, while lower values suffice for interactive use. 5.0 was used for the examples.
Similarly, the smoothness of molecular surfaces (not included in the examples) can be increased by increasing the vertex density in the surface attributes panel.
Straight edges may appear as tiny zigzags, especially against a white background. Supersampling gets rid of such jagged edges within saved images by making an initial image larger than that requested and then sampling it down to the final size. The level of supersampling can be controlled in the Save Image dialog. The default value of 3x3, used for the examples, is generally sufficient for publication.
Resize the Chimera window to the desired aspect ratio, and then find view(s) suitable for the images. For the example images, a position was found that showed the overall structures well, and when scaled up (without further rotation) showed the conserved side chains well. The zoomed-out position was named overall, the scaled-up position was named closeup, and a session file named images.py was saved in the working directory:
Command: savepos overallTo browse to a save location, use File... Save Session As instead of the command save.
Command: scale 4
Command: savepos closeup
Command: reset overall
Command: save images
In this example, the session was saved in the overall position, but it could have been saved in another position (including one without a name) without any loss of information. It is generally prudent to save sessions for publication images, as this decreases the labor necessary if figures have to be redone. Often minor adjustments such as changing a color or displaying a different set of side chains will be required.
Later, the session can be restarted by opening images.py. If the models are moved around, the saved positions can be regenerated, for example:
Command: reset closeup
Before an image is saved, a particular position may need to be restored, for example:
Command: reset overallThe overall and closeup positions were used for the examples.
Choosing File... Save Image brings up the Save Image panel. Entering a value for one dimension adjusts the other automatically when Maintain current aspect ratio is on. The dimensions can be specified directly in pixels, or in units of length (when Use print units is on). If units of length, the output pixel dimensions are calculated using the specified Print resolution.
For example, to make an image for a single-column width of 85 mm (3.346 in) with a resolution of at least 300 dpi, possible approaches include:
The example images were saved in TIFF format; other possibilities are PNG, JPEG, PS (PostScript), and EPS (Encapsulated PostScript).
Labeling, stereo, and color space are discussed in the Tips on Preparing Images. Images can also be created by raytracing with POV-Ray.